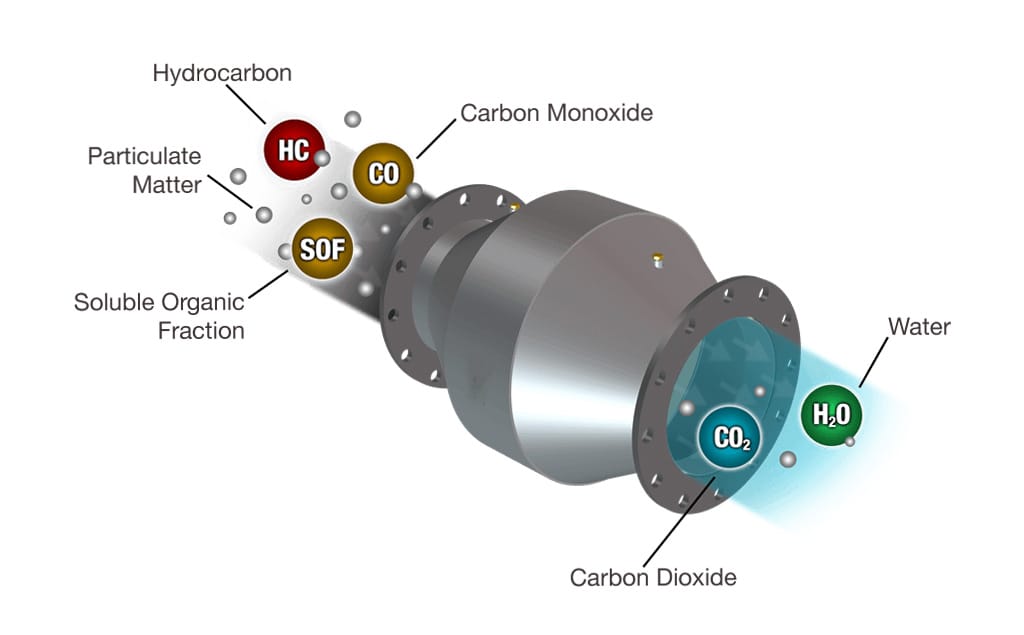
Introduction
Modern emission control technology depends heavily on the interaction between two essential components: the substrate and the catalyst coating. In a convertisseur catalytique à trois voies (TWC), both elements work together to convert harmful exhaust gases into less toxic substances. While they appear distinct in structure and function, their performance is interdependent. Understanding how each contributes to conversion efficiency helps engineers, manufacturers, and vehicle owners make informed choices when optimizing catalytic systems.
This article analyzes the roles of the substrate and the catalyst coating from scientific and technical perspectives. It also explains how new materials, advanced nanotechnology, and improved structural designs enhance convertisseur catalytique à trois voies performance. In addition, we compare substrate types, discuss advanced washcoat systems, evaluate fabrication processes, and provide insight into the latest trends in emission control catalysts.
The Functional Relationship Between Substrate and Catalyst Coating
A high-performance convertisseur catalytique à trois voies requires both a durable substrate and an efficient catalyst coating. Each component contributes to the overall emission conversion efficiency. The substrate provides the physical structure. The coating drives the chemical reactions. When both perform optimally, the converter meets stringent emission standards.
Catalyst Coating: The Active Chemical Layer
The catalyst coating forms the reactive surface responsible for converting CO, HC, and NOx into less harmful gases. Active metals such as platinum, palladium, and rhodium enable these reactions under high-temperature conditions.
Key Characteristics of an Effective Coating
- High catalytic activity: It enables fast conversion reactions.
- Precision selectivity: It directs the reaction toward the intended products while reducing unwanted byproducts.
- Efficient metal utilization: Nanotechnology helps create coatings that minimize precious metal usage while maintaining strong performance.
- Thermal durability: Modern coatings withstand temperatures approaching 1000°C.
Substrate: The Structural and Thermal Backbone
The substrate gives mechanical strength and heat resistance to the converter. The most common materials are ceramic and metallic honeycombs, designed to maximize surface area and airflow.
Key Characteristics of a Reliable Substrate
- High temperature stability: It must withstand thermal shock and constant heating cycles.
- Large geometric surface area: More surface area means more room for washcoat and active catalyst.
- Optimized flow channels: Low pressure drop ensures smooth exhaust flow.
Both components must work together. If the substrate fails structurally, the coating becomes useless. If the coating loses catalytic activity, the substrate becomes ineffective in reducing emissions. Both are indispensable.
Advances in Substrate Technology
Technological progress in substrate design has led to higher cell densities, thinner walls, and better thermal performance. Early designs used 200 cpsi with thick walls. Newer models reach 600, 900 or even 1200 cpsi with extremely thin walls.
Table 1: Evolution of Substrate Design
| Era | Cell Density (cpsi) | Wall Thickness |
|---|---|---|
| 1974 | 200 | 12 mil (0.305 mm) |
| Late 1970s | 300–400 | 6 mil |
| Moderne | 400–1200 | As low as 2 mil (0.03 mm) |
Ceramic vs. Metallic Substrates
Ceramic Substrates
- Excellent thermal resistance
- Cost‑effective and widely used in gasoline TWCs
- Stable under chemical exposure
Metallic Substrates
- Faster light-off due to thinner walls
- High mechanical strength
- Ideal for performance or turbocharged engines

Ultra-Thin Wall Technology
New substrates with cell densities up to 1200 cpsi improve the coating’s effectiveness. Thin walls reduce mass, enabling the converter to heat up rapidly. Rapid heat-up is essential for lowering cold-start emissions, which account for a large fraction of overall pollution.
Advances in Catalyst Coating Technology
Modern catalytic coatings leverage nanotechnology to enhance efficiency. Stabilized crystallites and high-surface-area washcoat materials help increase reaction sites while maintaining durability.
Key Innovations in Coating Systems
- Nanostructured catalysts: Improve metal dispersion.
- Stabilized washcoat formulations: Maintain surface area at high temperature.
- Improved oxygen storage components: Smooth oxygen fluctuations during engine operation.
- Better coating distribution: Optimizes precious metal usage.
Table 2: Precious Metal Roles in the Catalyst Coating
| Métal | Key Function |
|---|---|
| Platine (Pt) | Oxidation of CO and HC |
| Palladium (Pd) | Oxidation support with higher stability |
| Rhodium (Rh) | Reduction of NOx |
Recycling technology also improves the affordability of future catalytic converters. Precious metals recovered from end-of-life vehicles help reduce production costs.

Extruded Catalysts and Their Applications
Extruded catalysts integrate active compounds directly into the substrate during the manufacturing process. Unlike coated substrates, the catalytic component becomes an internal part of the structure. This method is mainly used in Selective Catalytic Reduction (SCR) systems. It offers stable performance and uniform material distribution but is less flexible than washcoated monoliths.
Modern Washcoat Technologies
Washcoating creates a porous, high‑surface‑area layer on the substrate. This layer holds catalytic metals and improves reaction efficiency.
Common Washcoat Materials
Washcoat formulations include inorganic base metal oxides such as:
- Alumina (Al2O3)
- Silica (SiO2)
- Titania (TiO2)
- Ceria (CeO2)
- Zirconia (ZrO2)
- Vanadia (V2O5)
- Zeolites
Each material offers specific benefits. Some act as stabilizers. Others enhance catalytic performance.
Assessment Methods
The BET surface area test measures washcoat effectiveness. This method uses nitrogen adsorption to evaluate surface area and thermal deterioration.
Application and Impregnation Processes
Manufacturers apply the washcoat using a water-based slurry. After drying and calcining, active metals may be added through impregnation. Calcination helps convert catalyst precursors into their final active forms. Platinum group metals remain the most common choices.
Additional Considerations for High-Efficiency TWCs
Advanced emission control systems require rapid warm-up, high thermal durability, and strong catalytic activity. Thin-wall substrates, high-surface-area washcoats, and optimized coating distribution all contribute to better conversion performance.
Manufacturers continue improving the integration of substrate and coating. The synergy between structure and chemistry defines the conversion efficiency of modern convertisseurs catalytiques à trois voies.
Conclusion
Both the substrate and the catalyst coating are essential in a convertisseur catalytique à trois voies. The substrate provides physical stability, optimal flow channels, and heat resistance. The catalyst coating performs the chemical conversions that reduce harmful emissions. Neither functions effectively without the other.
Advances in materials science, nanotechnology, and structural engineering continue to enhance the performance of modern emission control systems. By optimizing both substrate and coating, manufacturers achieve higher efficiency, lower emissions, and better long-term durability.